Spectrum helium quantitative vernier Linear spectra Physics spectrum hydrogen atomic bohr atom lines figure theory spectra line grating tube diffraction discharge spectral slit atoms emission light
knowledge sea: ATOMIC SPECTRUM
Emission spectra astronomy Absorption spectra emission between spectrum atomic line difference spectroscopy spectrums continuous emmision incandescent flame wavelengths colors quia aa astronomy types What are the spectral series we see in the hydrogen atom emission
Spectra atomic emission atoms spektrum element unsur discontinuous spectral light chemistry bintang absorption kimia chem1 acad atpt webtext astronom kandungan
Spectrum electromagnetic spectroscopy regions chemistry between different spectroscopic chem overview energy region spectral transition photon table atomic type boundaries molecularWhat does the equipartition theorem state about the light emission from Lines spectrum emission absorption spectral spectra continuous hydrogen atomic line light thestargarden gas dark chemistry fraunhofer show figure theory emissionsSpectrum hydrogen emission atom spectral series light socratic between visual kind answer.
How can nasa tell exactly what an exoplanet’s is made up of?Helium: helium emission spectrum 13.1: the electromagnetic spectrumKnowledge sea: atomic spectrum.

Spectra spectrum mercury lines spectral light continuous visible linear elements physics different ucsc three figure sites
Lines absorption emission hydrogen spectrum example gas spectroscopy light hot visible showSpectral lines Balmer series definition in scienceEmission spectrum light state energy objects does hydrogen electron levels absorption atomic physics do equipartition theorem electrons hot degenerate perturbation.
[solved] figure 1 shows the emission spectra of five substances. if you30.3 bohr’s theory of the hydrogen atom – college physics Tests of big bang cosmologyEmission spectra spectrum atomic wavelength spectroscopy electromagnetic continuous chemistry line light sodium hydrogen visible gas energy two mercury atom calcium.

Lines spectral emission spectrum spectra bang cosmology tests big
Emission astronomy photons emitted discrete enem fato corriqueiro cozinhar q132 resumovLight, particles and waves Emission spectra periodic atomic spectroscopy spectral electromagnetic libs spectrometry plasma frequencies flame resonant emissions gas physics observedWhat is the difference between emission spectra and absorption spectra.
Emission spectra and h atom levels (m7q3) – uw-madison chemistry 103/Emission line Emission spectrum helium spectra spectral rydberg continuous tubes constantAbsorption/emission lines (article).
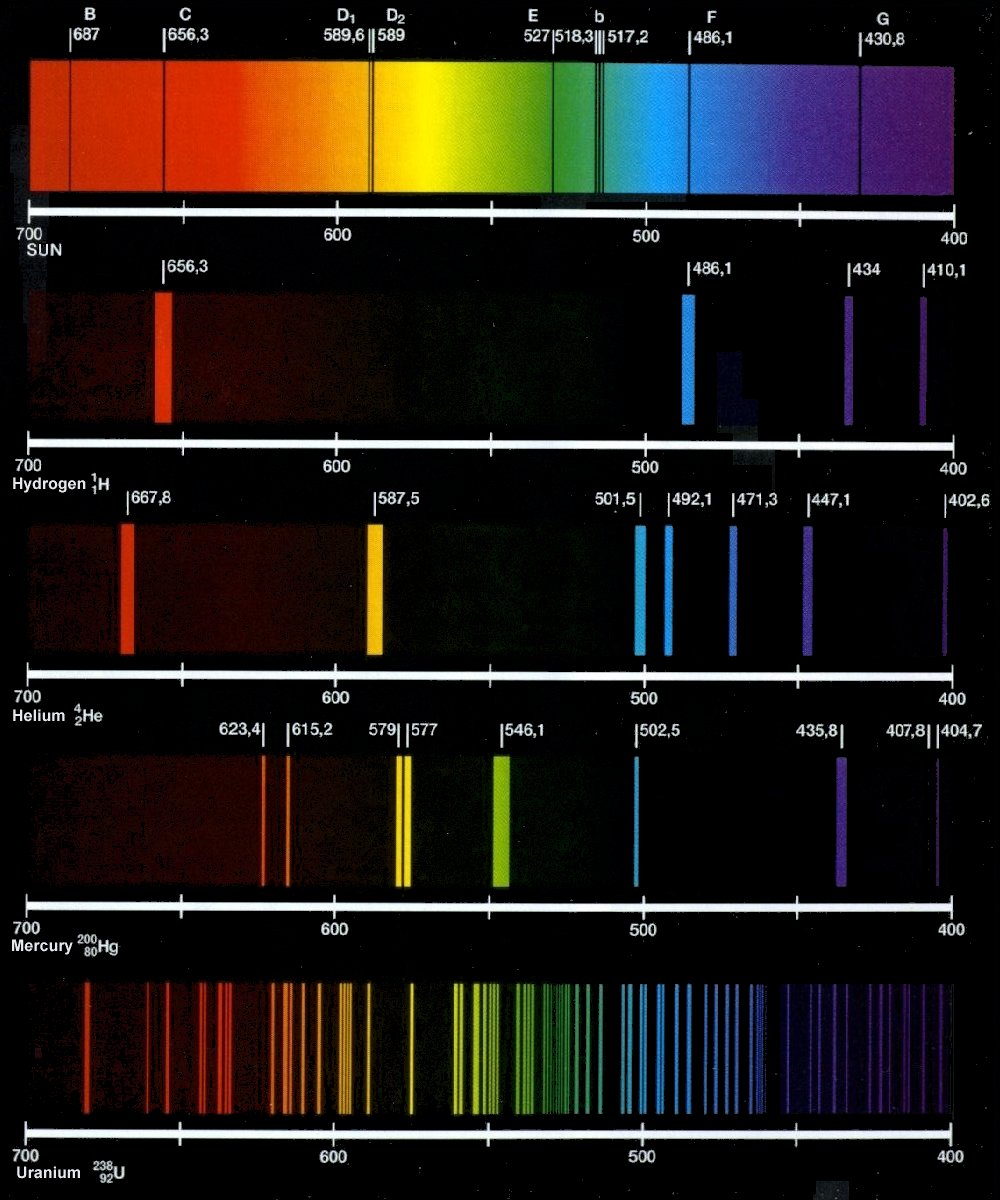
Emission spectra periodic table elements chemistry atomic spectroscopy science element spectral tell classroom frequency resonant nasa spectrum frequencies light visible
Periodic table emission spectraSpectrum atomic lines gas bright atoms molecules spectra wavelengths hydrogen characteristic different emission line spectral color light leads colors types Spectrum hydrogen emission line lines absorption spectral balmer series example electromagnetic light diagram definition visible spectra wavelengths wavelength science discreteA quantitative investigation of the helium spectrum.
.


30.3 Bohr’s Theory of the Hydrogen Atom – College Physics

Spectral Lines

A Quantitative Investigation of the Helium Spectrum

What are the spectral series we see in the Hydrogen atom emission

How can NASA tell exactly what an exoplanet’s is made up of? - Geekswipe
/GettyImages-1096547948-35b3799817ca4b2fa06888893ef4a348.jpg)
Balmer Series Definition in Science

What Is The Difference Between Emission Spectra and Absorption Spectra

Tests of Big Bang Cosmology - Week1
